Ensuring the safety and reliability of medical device software is paramount. Static analysis plays a crucial role in identifying potential vulnerabilities early in the development lifecycle. This article explores the importance of static analysis in healthcare, examines regulatory frameworks, and highlights best practices for implementing software tools for static analysis.
Software defects in medical devices can have life-threatening consequences. Static analysis, a method of debugging by examining source code before a program is run, helps identify these defects early, reducing the risk of harm to patients and costly recalls. By automating code inspection, static analysis tools enhance development efficiency and improve overall software quality.
Regulatory bodies like the Food and Drug Administration (FDA) and the European Medical Device Regulation (MDR) mandate rigorous testing for medical device software. Static analysis tools assist in meeting these stringent requirements by enforcing coding standards (e.g., MISRA, CERT) and detecting potential vulnerabilities like buffer overflows and null pointer dereferences. Compliance with these standards is essential for market approval and demonstrates a commitment to patient safety.
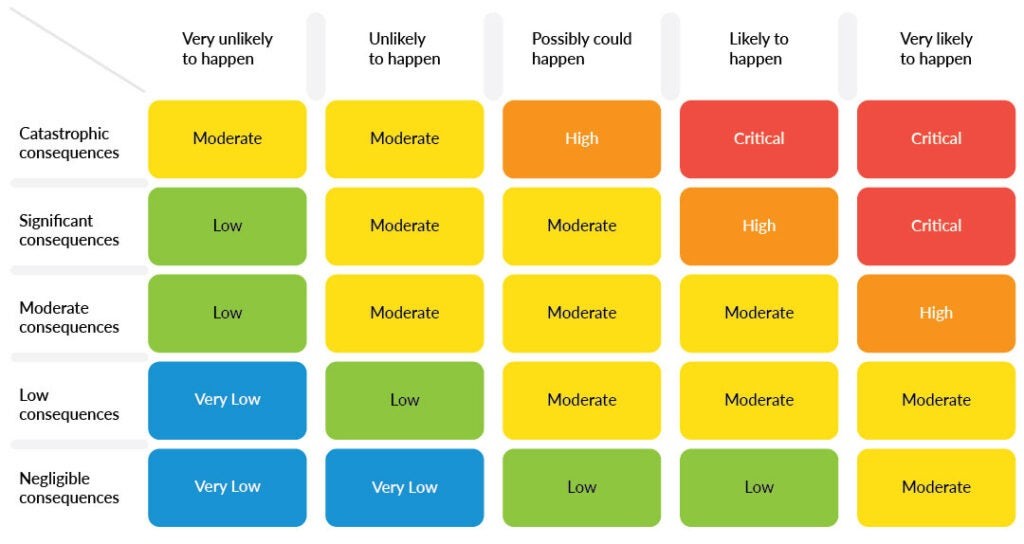 Table graphic showing safety integrity level (SIL)
Table graphic showing safety integrity level (SIL)
Example of a safety integrity level (SIL) table demonstrating risk categorization in medical device software.
Implementing Static Analysis Tools: Best Practices
Integrating static analysis into the development workflow requires careful planning and execution. Here are some key best practices:
Early Adoption and Integration
Introduce static analysis tools early in the development process. This allows for continuous code inspection and prevents the accumulation of technical debt. Addressing issues as they arise is significantly more efficient than tackling a large backlog of errors later in the project.
Compiler Optimization
Utilize compiler warnings effectively. Treat warnings as errors to ensure that potential issues are addressed promptly. Configure compilers with strict settings (e.g., -Wall, -Werror in GCC) to maximize the detection of potential problems.
Prioritize Critical Violations
Initially, a static analysis tool may flag numerous violations. Prioritize fixing critical issues first, focusing on those that directly impact safety and security. Establish a clear process for managing and resolving less critical violations over time.
Customize Rule Sets
Tailor the rule sets of the static analysis tool to the specific needs of the project. While default rule sets provide a good starting point, customizing them allows for a more focused analysis and reduces the number of false positives. Regularly review and update these rules to ensure they remain relevant.
Example of a static analysis tool highlighting potential vulnerabilities in code.
Complement with Dynamic Analysis
Combine static analysis with dynamic analysis techniques. Dynamic analysis examines the running code, providing insights into runtime behavior and memory management. Using both methods offers a more comprehensive approach to software verification and validation.
Tool Qualification and Validation
Ensure that the chosen static analysis tool is qualified and validated for its intended use in the medical device development process. This is a regulatory requirement and demonstrates the reliability of the tool’s results. Parasoft, a leading provider of software testing solutions, offers tools and resources to streamline this process.
Conclusion
Software tools for static analysis are indispensable for ensuring the safety and reliability of medical device software. By adhering to best practices and integrating these tools effectively into the development lifecycle, healthcare organizations can significantly reduce risks, improve software quality, and comply with stringent regulatory requirements. The investment in static analysis translates to safer patient care and greater confidence in the reliability of medical devices.


